Healthcare & Pharma
The Healthcare industry spans multiple segments, such as the pharmaceutical, biotechnology, healthcare provider, child clinics, healthcare insurance, and research segments. All these contribute directly or indirectly towards patient care or drug development. Y&L brings industry expertise covering a variety of manufacturing and retail nuances. We understand manufacturing criticalities, procurement, production, shop floor control, sales, customer management, quality, and supply chain management.The focus is to improve operational efficiencies, reduce risk and cost, and improve customer service. Y&L provides a wide range of business consulting, systems implementation, and process management services to its healthcare clients.
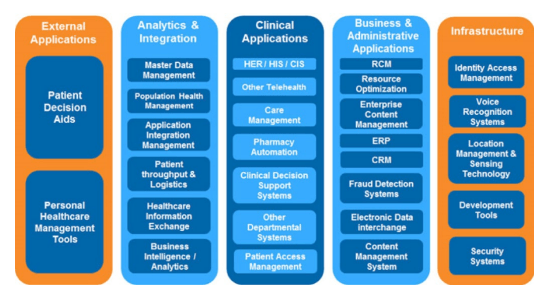
Y&L helps healthcare organizations improve their IT architecture to support and adapt to constant changes and provides value-added services to help manage healthcare facilities as enterprises and provide decision support systems. In addition to application development, Y&L also provides much-needed network infrastructure support in terms of
creating Wi-Fi hotspots and facility connectivity to allow physicians to work wirelessly on tablets to update patient records. Ask us about the work we have done for BlueCross/BlueShield, Thermo Fisher Scientific, Vidacare, PharMerica, United Allergy Services, CaptureRx, Wellmed, Onco 360, Nightingale Home Healthcare Services, Boxhill Hospital, Shriner’s Hospital, BioBridge Global, HVHC, Mission Pharmacal, Southwest Research Institute, Charles River, Smith & Nephew and Merck.

Y&L SAP Pharma Rapid Deployment Solution (RDS)
Based upon extensive experience, Y&L has developed a pre-configured Rapid Deployment Solution (RDS) for SAP Pharma based upon our industry best practices and contains a built-in GxP control specifically for pharmaceutical companies. Y&L’s Pharma RDS solution can help reduce your Total Cost of Ownership and regulatory compliance risks, while improving quality management and innovation.
PharmaOne
PharmaOne is a comprehensive, prepackaged SAP® Business All-in-One ERP solution designed specifically for the pharmaceutical manufacturing industry. Leading pharmaceutical and biotechnology manufacturers are leveraging PharmaOne to improve operational efficiency, increase employee productivity, and respond to market demands with agility.
PharmaOne delivers the necessary tools to improve business performance:
- Adherence to FDA compliance
- Track and manage plant-wide data with ease
- Tight integration with finance and improved cost visibility
- FIFO-FEFO batch determination, shelf life monitoring, traceability and Electronic Batch Record (EBR)
- 102 GxP In-built controls
Computer Validation Services for FDA Compliance
Organizations in the life sciences industries must comply with the Computer System Validation (CSV) requirement that is issued from the FDA. The regulation mandates that companies in the life sciences industries have the specific controls and procedures in place to consistently produce results that meet its predetermined specification and quality attributes.
Y&L brings a thorough understanding of this requirement and a practical risk-based approach to CSV and helps enable life science customers to meet global regulatory requirements, eliminating the GxP compliance risk. Our highly qualified and experienced consultants have real-world domain experience and have the right capabilities and tools to tackle ERP, manufacturing, laboratory, and clinical systems.
Y&L’s CSV compliance services include:
End to End computer System Validation Servics
Offered for any GxP sensitive computer system implementation, development, upgrade, and rollout project, services include: IQ, OQ, PQ with all other validation deliverables as per GAMP 5 standards. The services also include GxP Control Mitigation and 21 CFR Part 11 Analysis. Y&L has standard approved CSV templates, processes and procedures, tools, reusable assets like User Requirements, Functional Requirements, etc. which helps reduce the CSV timelines.
21 CFR Part 11 Analysis and System Remediation
21 CFR Part 11 Analysis and Remediation is conducted as part of CSV end to end validation services or separately if 21 CFR Part 11 compliance is not implemented. 21 CFR Part 11 is also referred to as Electronic Records and Signatures and is mandatory for FDA regulated industry to comply.
GxP Risk Assessment and Control Mitigation
GxP Risk Assessment and Control Mitigation is conducted as part of CSV end to end validation services or separately if GxP Controls are not implemented or found missing during CSV audit. Y&L has a robust GxP Risk Assessment Tool to enable, track and incorporate GxP Control Requirement in the GxP System.
Validation Testing Services
Validation Testing Services is an independent service where Y&L provides functional resources with the relevant functional and CSV expertise to execute validation testing of GxP System and ensure objective evidence in form of proper documented evidence adhering to Good Testing and Documentation Best Practices. Validation Testing Services can be offered as part of CSV end to end services or a separate service and can also be executed 100% offshore.
Quality Assurance Review
Quality Assurance (QA) Review service is an independent service offering for review of all Validation deliverables. QA Review service can also be offered during Validation Testing with independence of Review maintained if Y&L is also performing Validation Testing Services.
CSV Audits
CSV Audit is conducted periodically preferably annually to check the compliance health of Validated and Non Validated GxP System. Y&L will conduct the CSV Audit and generate a CSV Audit report along with mitigating steps and procedures. Y&L can also help customers to close on compliance gaps.